您现在的位:首页 > 新闻动态 > 公司新闻 > 公司新闻
Simple Western用于创新药物临床试验pharmacodynamic(PD)生物标志物检测
来源:东岱科学器材日期:2018-06-04 14:32:00浏览次数:次
2010年美国NCI研究药物指导委员会生物标志物工作委员会(The Biomarkers Task Force of the NCI Investigational Drug Steering Committee)发布了新药早期临床试验生物标志物研究开发指南:Guidelines for the Development and Incorporation of Biomarker Studies in Early Clinical Trials of Novel Agents.
各类Biomarker定义及作用
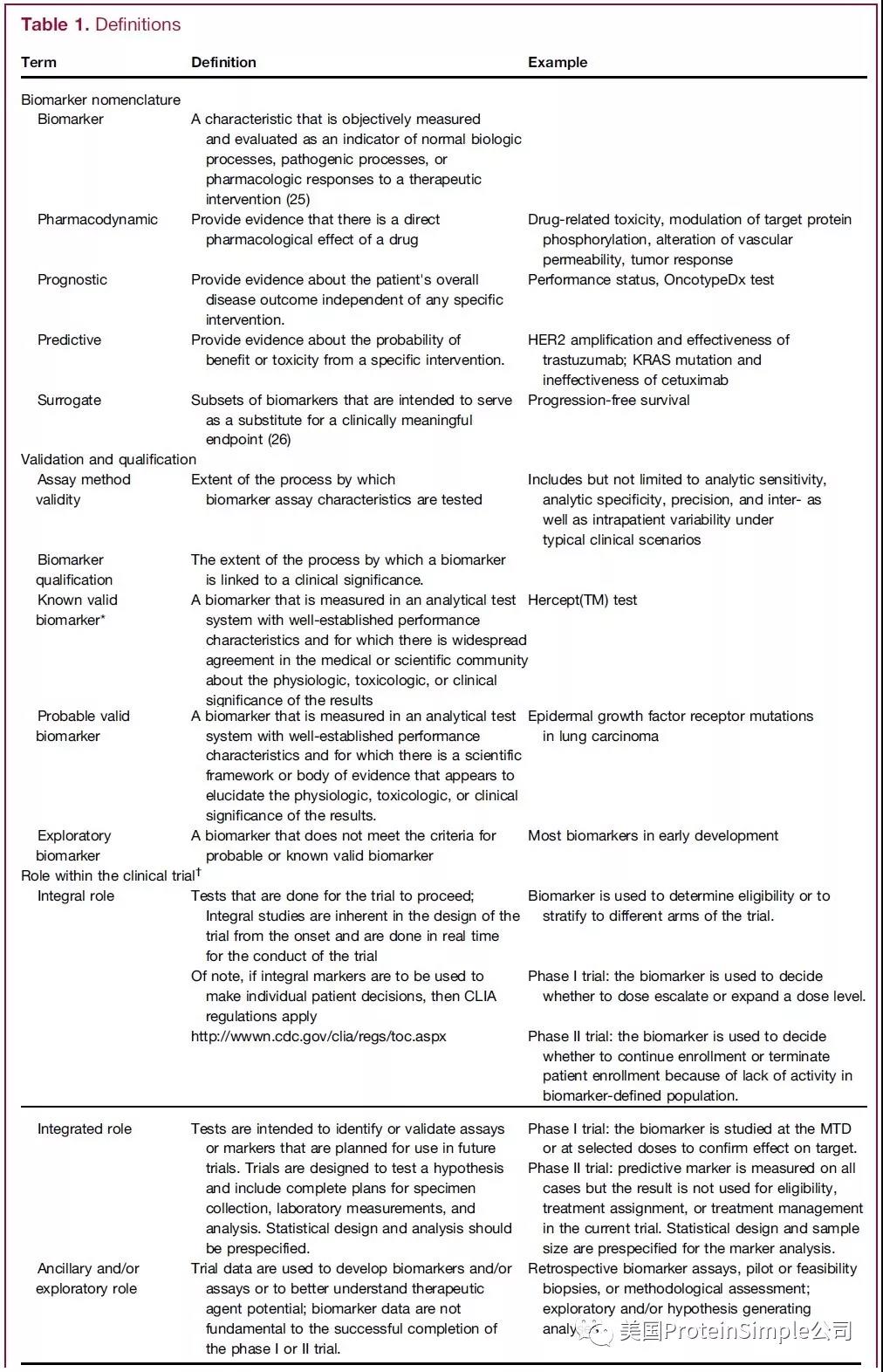
其中pharmacodynamic(PD)提供药物是否有作用的直接生物学证据及毒理学基础信息,因而美国很多创新药物临床一期和二期中选择合适的pharmacodynamic(PD)生物标志物,用于药物在人体生物学效应监测。
文中谈到Biomarker研究和检测的三个考量因素:
1. 标志物科学性:Strength of science
2. 实验稳定性:Assay robustness
3. 实验可行性:Assay Feasibility
案例一: 阿斯利康 AZD1208 全球多中心,开放标签,First-in-human
2018年5月Nature出版集团British Journal of Cancer在线发表了,阿斯利康AZD1208,一种原发性整合型莫洛尼病毒激酶抑制剂,在血液肿瘤和实体肿瘤的全球多中心,开放标签,临床一期实验数据。本次临床研究包括全球多个权威肿瘤中心:MD Anderson Cancer Center血液肿瘤科,日本National Cancer Center Hospital乳腺肿瘤科,美国Dana-Farber Cancer Institute 肿瘤科,英国Royal MarsdenHospital 前列腺癌靶向治疗和药物开发部,加拿大玛格丽特公主医院Princess Margaret Hospital 安大略癌症研究所,华盛顿大学医学院肿瘤中心等。
研究目的:首要目的:AZD1208在急性髓细胞白血病(AML)(ClinicalTrials.gov, NCT01489722)和advanced solid malignancies(NCT01588548),剂量递增研究,每日口服一次的最大耐受剂量,药物安全性和耐药性。次要研究目标:药物PK和有效性的初步证据,及反应药效pharmacodynamic(PD)的生物标志物。
Study objectives
Both dose-escalation studies recruiting patients with AML (ClinicalTrials.gov, NCT01489722) and advanced solid malignancies (NCT01588548) were phase I, open-label, multicentre studies designed to identify the maximum tolerated dose (MTD) and evaluate the safety and tolerability of AZD1208 administered orally once daily (QD). Secondary objectives included evaluation of the drug PK and preliminary evidence of efficacy. The AML study also explored pharmacodynamic (PD) biomarkers.
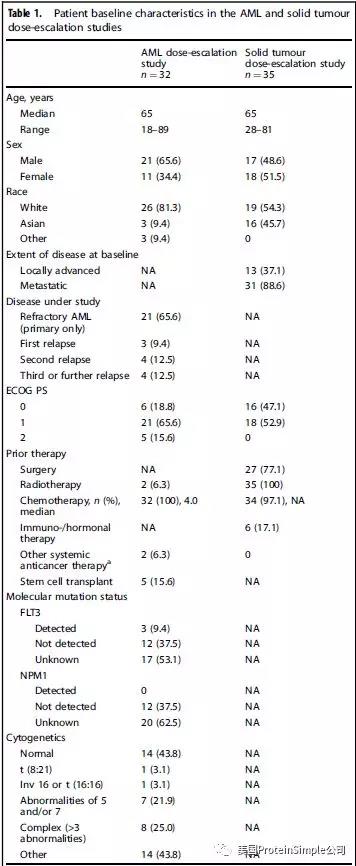
入组病人基本资料
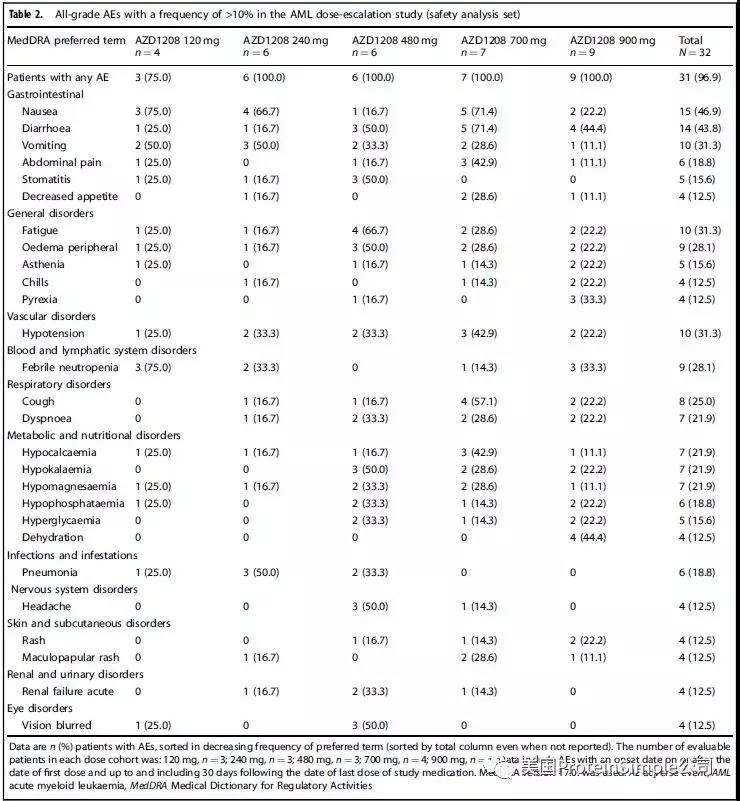
剂量递增安全性分析
药效pharmacodynamic(PD)作者选用Simple western(Nanopro1000)分析protein level changes of phosphorylated 4E-BP1 S65

案例二:KB004, a first in class monoclonal antibody targeting the receptor tyrosine kinase EphA3 一期临床研究
2016年Leukemia Research发表了,KaloBios PharmaceuticalsKB004,一种靶向EphA3的单克隆抗体,在恶性血液病,全球9个中心,开放标签,临床一期实验数据。本次临床研究包括全球多个权威肿瘤中心:MD Anderson Cancer Center血液肿瘤科,迈阿密大学血液肿瘤科,斯坦福大学肿瘤所,澳大利亚阿尔弗雷德医院和莫纳什大学,澳大利亚皇家布里斯班妇女医院,美国克利夫兰诊所,澳大利亚韦斯特米德医院,澳大利亚皇家阿德莱德医院,美国H.Lee Moffitt癌症中心。
药效pharmacodynamic(PD)作者选用Simple western(Peggy Sue,NIA)系统分析分选微量CD34+细胞 EphA3表达。
Pharmacodynamic (PD) assessments were performed at baseline and serially thereafter, to determine the degree of EphA3 expression on tumor cells CD34+ cells were isolated from marrow samples by immune-magnetic separation and cryopreserved at −80C for batch analysis . Subsequently, cells were thawed, washed and immediately lysed for NIA analysis. Equal numbers of cells for each sample were loaded for analysis into the NIA instrument (PeggySue system, ProteinSimple, Santa Clara, CA). In the instrument, proteins from each sample were separated based on charge using capillary electrophoresis. EphA3 was detected using E11F12 antibod
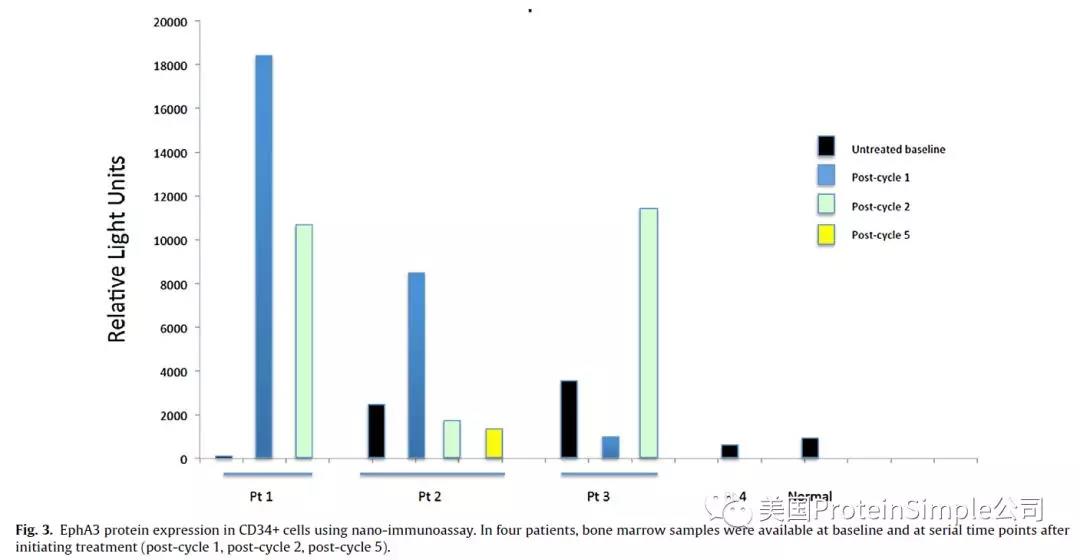
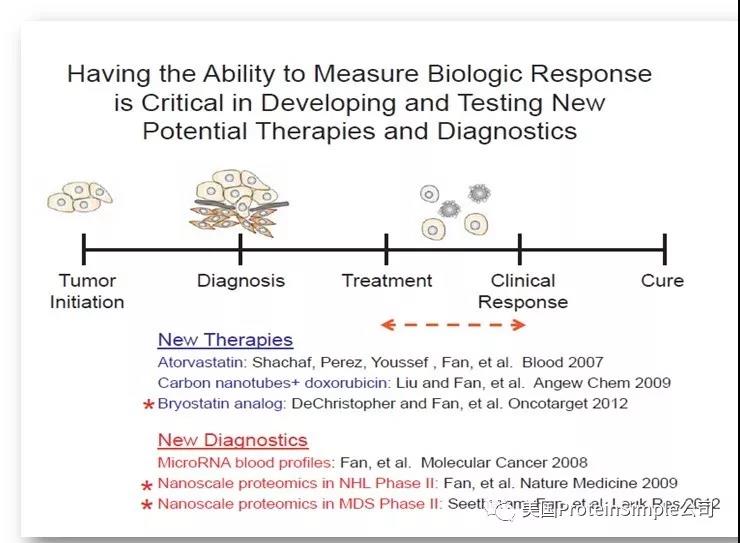
之前,Simple Western还在多个临床试验中被用于监测Biological Response。




 电话
电话 主页
主页 留言
留言